Explained: The Value of Residual Kidney Function to Dialysis

A home haemodialysis patient from New Zealand asked the Home Dialysis Central Facebook discussion group: “Why do dialysis patients have different outcomes with regard to maintaining residual kidney function?” And, “Why do some people seem to have an easier time than others with the control of salts like sodium, potassium, and phosphate?”
My first response must be that while the functions of the normal kidney are well known, the myriad variable and interrelating dysfunctions of kidney physiology (or, functional pathophysiology) that occur once dialysis begins, are not. Indeed, I almost despaired of making any sensible contribution as few (if any) studies have looked at the contribution residual renal function makes (if any) to electrolyte regulation in dialysis patients!
But, home patients (especially) are an inquisitive lot. They often ask interesting questions as they seek to understand the unknown. So, for them, I resolved to give it my best shot. My answer has grown into a gargantuan beast, but, here goes…
Residual Renal Function (RRF)
RRF is a term often used to describe the remaining contribution, or contributions—note the plural—the last remnants of failing kidney tissue may still make to overall water and waste clearance, once dialysis has begun.
It can seem contradictory to patients, especially home patients who are struggling to understand the therapy they are self-applying, that despite being told they need dialysis, they may still be passing what seems like plentiful urine. On the other hand, some do not pass much urine. Why the difference?
RRF – to patients – can mean different things. Some interpret it to predominantly signify preserved (or lost) urine output. Others think of it in terms of clearance of wastes. Still others, a combination of the two. In truth, all are correct, depending on the circumstance.
How Kidneys Work
Our kidneys make urine, which provides our physiology (normal body function) with two basic things:
- A balance and control of the exact amount of water and right concentration of the salts (electrolytes) that fill and bathe our cells, structures and organs. Electrolytes are simply chemicals that, when dissolved in water, are positively or negatively charged (like a battery) and can transmit electricity. Common electrolytes include sodium, chloride, potassium, calcium, phosphate, magnesium, bicarbonate, and many others. By losing or retaining water, and by excreting or reclaiming electrolytes, the kidneys finely regulate and adjust the volume of water and the concentration of salts in body tissues.
- They filter away (or “clear”) all the waste substances that our cells, structures and organs make in their daily functioning. This is why the term “clearance” is used for the excretory function of the kidneys.
To perform these two functions—water and electrolyte balance, and waste clearance—the kidneys have evolved an exquisite mechanism: the nephron.
Each kidney contains an average of about one million nephrons, though the range between individuals and racial origins is very wide. For example, Australian aboriginals tend to be nephron poor, having as few as 350,000 nephrons per kidney, while some Caucasians may have as many as 1.5 million per kidney.
Low nephron number has many possible causes, some of which appear genetically determined, but nephron number may be key to the ability to withstand or cope with kidney disease. It may be one of the underlying reasons why some people (perhaps with a higher nephron number) seem more resilient to the same “hit” of the same disease than others (who perhaps have a lower nephron number). One day, when we can accurately count nephrons, we may have a tool to aid in outcome prediction…but this is not yet practically possible (except at autopsy!).
Different Parts of Kidneys Do Different Tasks
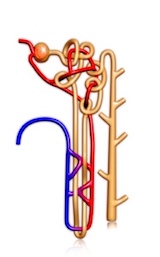 Each human nephron has two distinct parts:
Each human nephron has two distinct parts:
A glomerulus (a leaky filter)
A tubule (a filtrate modifier)
Simplistically, glomeruli (= the plural of glomerulus) filter solutes (electrolytes + wastes) and water out of the bloodstream as blood passes through the kidneys. They “sweat” a fluid and solute mix across their leaky membrane walls—a bit like those leaky black garden hoses do.

Staggeringly though, more than 180 liters of water and solutes “sweat” across the glomerular membrane and into the tubules every 24 hours. That is a fluid volume equal in weight to about 2 ½ times the body weight of an average human! Clearly, we would not live more than a few minutes if that enormous amount of fluid were to pass, unaltered, as our final urine. Something magical must happen to it as it passes through the tubular system – allowing the body to grab back most of the water and salts, but allowing continued waste excretion.
The tubules must reabsorb almost all the fluid that poured through the glomeruli, and leave a mere 1.5 – 2.0 liters to be passed as the final fluid we call urine. But, that urine must still contain all the solute wastes that must be eliminated. The renal tubule—one for each glomerulus—is a very smart and complicated structure!
The tubules are a frantic two-way interface. While some things are being reclaimed from the tubular fluid, others are being exchanged and/or excreted. Think of a post office: letters and parcels of all shapes and sizes in, letters and parcels of all shapes and sizes out.

The renal tubule is a hive of industry. Tubules suck back (reabsorb) all but 1.5 - 2.0 of the 180+ liters that are unselectively washed through the glomerulus each 24 hours:

Most of the filtered sodium is returned to the bloodstream.
Potassium, calcium, magnesium, bicarbonate, glucose and most proteins are reabsorbed.
Additional creatinine and uric acid (urate) is excreted.
Urea is reabsorbed to help reclaim water.
While the glomeruli provide a non-selective waterfall of fluid and solutes, the tubules adjust this torrent to ensure that the final urine is volume and content perfect. Water, glucose, and electrolytes are balanced for physiological need—but the urine is waste-rich to ensure that as much waste is eliminated as possible.
There are, broadly, four different sections in each renal tubule—with each section performing a different set of tasks. Tubular function is regulated by a raft of osmotically-active hormones: anti-diuretic hormone (also known as vasopressin), natriuretic hormone, aldosterone, and others. Lots of energy-requiring chemical pumps inhabit the cells that line the tubules, reading, adjusting, pumping and transporting substances into and out of the tubular fluid to ensure the internal chemistry of the blood is kept “just so.”
Again, renal tubules are very complicated structures!
Why Are There Different Responses to Kidney Disease?
When kidney diseases strike, nephrons can be damaged in different ways and at different sites along their length. This depends on the disease process (there are scores of kidney diseases), and on the part (or parts) of the nephron that are damaged by each disease:
Some diseases predominantly damage the glomerulus.
Others may cause more damage to tubular cells and structures.
Many do both—with varying degrees of scarring and fibrosis (a gristly thickening of the cellular tissues) affecting glomeruli, tubules, and the interstitium of the kidney—the part that occupies the space between nephrons, supporting them, and providing a two-way pathway between the tubules and the microcirculation of the kidney.
But, when one part of the nephron is damaged, it affects other parts, too, even if those are spared direct damage. And, as nephrons are damaged, their function is compromised, altered, or completely lost.
Damage can occur at different rates, too. Tubular damage may occur at different parts of each tubule, and thus affect different functions differently. And, to make it even harder, damage and cellular death can be very patchy, with some parts of the kidney being relatively spared while other parts are being damaged beyond recognition. So, even the same disease can damage different people in different ways.
Why Does CKD4 Eventually Progress to Dialysis?
I use this example with my patients:
Imagine a factory making a product: any factory, any product. It employs 100 workers. The bean-counters come in and make 50 workers (50% of the work force) redundant. The other 50 would work some overtime, grumble now and then, but still keep the factory running and producing its product, even if it means working a little harder.
 But, then they fire another ½ of the work force. Now, the factory is down to only 1 in 4 of its original staffing. A point is reached where—even with all the goodwill in the world, with all the harder work—the product just cannot be made. The factory output begins to fall, and it is no longer profitable. Its workers begin to get sick, just from overwork, and leave. Still more may choose to leave. As more and more fall ill, or leave, the strain on those remaining gets more, and more. The factory is no longer sustainable. It closes down.
But, then they fire another ½ of the work force. Now, the factory is down to only 1 in 4 of its original staffing. A point is reached where—even with all the goodwill in the world, with all the harder work—the product just cannot be made. The factory output begins to fall, and it is no longer profitable. Its workers begin to get sick, just from overwork, and leave. Still more may choose to leave. As more and more fall ill, or leave, the strain on those remaining gets more, and more. The factory is no longer sustainable. It closes down.
This is just how it is with kidney function and kidney failure. Lose half, and the other half will cope. But, lose 3/4, and there is not enough left to sustain long-term function. While some more may be lost by disease, other still-functioning nephrons begin to “pack it in” from overwork. A point of self-fulfilling failure has been reached. Kidney failure becomes inevitable.
Back to Residual Renal Function
RRF – more particularly, residual urine output—matters, for several reasons:
In peritoneal dialysis patients, where solute clearance is not quite as efficient as that which is normally provided by haemodialysis, even severely damaged kidneys can still contribute important—even if small—additional solute removal.
In both modalities—though, this time, more importantly in haemodialysis—a sustained urine output means a freer, more comfortable fluid intake, and easier fluid management. Clearly, if urine output can be preserved, then both the ultrafiltration volume and the rate of ultrafiltration (fluid removal) can be—must be—correspondingly reduced. While ultrafiltration does increase solute drag and thus solute removal, this effect is not necessary for solute removal…it simply enhances it—but only to a small extent.
But…the home haemodialysis patient who maintains a good urine output is in an enviable position. At home, sessional length can be easily extended. The clearance gained from a longer session more than makes up for any (small) loss of clearance that may result from a lower or isovolaemic (= no change in net volume) ultrafiltration (UF) rate. Fluid management can be kept far more comfortable. I have read that some home patients are being told they must ultrafilter a stipulated minimum amount. But, if their urine output is sustained, their interdialytic weight gain is small, and their blood pressures are normal, their UF rate can and should be adjusted downwards, relative to sessional length, and the amount of fluid that needs removal to return to target weight. They ought not be illogically forced to maintain some arbitrary “prescribed rate.” The whole point of home haemodialysis dialysis is the immeasurable advantage that a patient can gain from lengthening the session. A longer session permits a lower rate of fluid removal, to the lasting benefits of both well-being and survival.
A low UF rate reduces or even completely negates any contraction of the blood volume (= isovolaemic dialysis). A stable blood volume will ensure that the blood pressure will remain stable and hypotension will be prevented.
Preventing in-treatment hypotension will ensure that organ perfusion will be sustained throughout the dialysis session: not only sustaining myocardial perfusion (a happier heart), cerebral perfusion (a less fogged brain), and gut perfusion (a less grumbly tummy), but will ensure preserved perfusion of the residual kidneys. This will ensure that urine output is not “turned off’ by the repeated and aggressive blood volume contraction that accompanies high ultrafiltration rate dialysis.
And so, the circle is completed. A sustained urine volume is preserved. Easy ongoing fluid management results, and residual renal function is sustained.
While this does not fully answer all of the questions the patient asked—for there really are no certain answers to all his questions—it does explain why any dialysis prescription that can lower the hourly UF rate is beneficial.
The data tells us that the lower the UF rate, the better: provided that a compensatory increase in session time is also prescribed to counterbalance any (small) loss of UF-driven clearance. It is all about long, slow, and gentle. Short, fast, and hard is just not correct. And…the sooner we learn this lesson, the better.


Comments
Brad
May 05, 2023 9:41 PM
Thanks in advance
Brad
May 06, 2023 11:27 AM
Stephen Noonan
Apr 01, 2022 9:46 PM
Beth Witten
Apr 02, 2022 11:18 PM
https://lifeoptions.org/learn-about-kidney-disease/slowing-kidney-disease/
Did you know that when your GFR is 20, you can be evaluated for and may be able to get a kidney transplant before dialysis, especially if you have a living donor? If you want to find out more about kidney transplant, talk with the transplant program in your insurance plan's network (U.S. transplant programs accept Original Medicare, but other insurance may not). Check out kidney transplant programs on this site. Look for "kidney" and enter your zip code. Adjust the distance you're willing to travel (50 miles is the default). You can look at wait times and outcomes at programs where your insurance will cover you.
https://www.srtr.org/
Roger G Horton
Apr 07, 2020 11:42 AM
I’m 58 yrs old, 220lbs, 5’10”. I just began dialysis 8 months ago, eGFR 7 and creatinine of 9. Fistula completed 1.5 yrs ago. My issue is that my dialysis clinic insists on wanting to take liquid off of me even though I still urinate. Yes I’ve been having some weight gain, but it’s not from fluid retention, it’s from an increased appetite, evidently from reducing my creatinine. I was pretty sick for about a year before I began dialysis, extreme nausea, headache, vomiting. Once I began I feel 1000% better.
But after dialysis I have about a 6 hour recovery time where I’m having some severe symptoms. My head feels like it wants to pop off, my face tingles, my heart is pounding, and I’m short of breath. I have limited them to only take off 1000ml now, but before they would take off as much as 3000ml, and I was having cramps 25% of the time. I’m just so tired of arguing with them, trying to get them to understand that all weight gain is not fluid retention. My ankles are not swollen, my lungs are clear, no signs or fluid build up at all.
The times when they have set the UF to just 500 or zero, basically no fluid removal, my after dialysis symptoms are minor. So I know that by them being so adamant about fluid removal for those who still urinate is hurting my body. What studies or evidence can you give me so that I can make them comprehend that all weight gain does not require fluid removal?
Thanks, Roger
Jeff Strachan
Feb 02, 2019 11:22 PM
John Agar
Feb 03, 2019 7:20 AM
Some patients even need fluid replacement during dialysis ... ie: are in negative fluid balance and need a ‘negative’ UF = amounts to fluid replenishment by and during dialysis. The idea that all patients require a minimum ‘x’ of UF - irrespective of native volume status - is incorrect.
True, most dialysis patients are, and remain, chronically fluid overloaded. But it is NOT true that this encompasses all. Individual staus is crucial. No two patients are alike, and blanket ‘rules’ for UF (especially) are inappropriate.
Enough said.
Angel B.
Jan 21, 2019 4:45 AM
Leong Seng Chen
Jan 12, 2019 10:51 AM
Thank you very much Dr John Agar!
John Agar
Jan 05, 2019 4:24 AM
Even CKD4 patients, while clearly at highr risk of eventual progression, may not ... or may progress so slowly that initiating dialysis of any ilk for their often stable, and usually symptom-free CKD (even if advanced CKD4) may (or more likely would) introduce significant dialysis complications with questionable (if any) benefit, and with unquestionable risk and treatment morbidity.
A strong argument can be made that dialysis is often started far too early in many US jurisdictions ... it is rarely needed above eGFRs of 8-10 ... and even then, an eGFR number is NOT the right way to determine the right moment to initiate dialysis.
Peter Laird
Jan 04, 2019 4:19 AM
Debra Null
Jan 04, 2019 1:59 AM
Thanks again!
Debra