High Fistula Blood Flow Rates and Microbubble Formation

In the past I have blogged on this site about my vehement opposition to the extraordinarily high blood flow rates so often recommended by (and practiced in) US dialysis services. In the following referenced past blogs – and in several others – I have focused on:
The risks of a high negative arterial “suck” pressure on the feeding arterial segment of the fistula
The risks of a high venous return pressure and the impact of the turbulence this creates in the venous return segment [1, 2]
The futility of using excessive blood flow rates to achieve improved solute clearance [2]. This graph (repeated from blog 2) says it all!
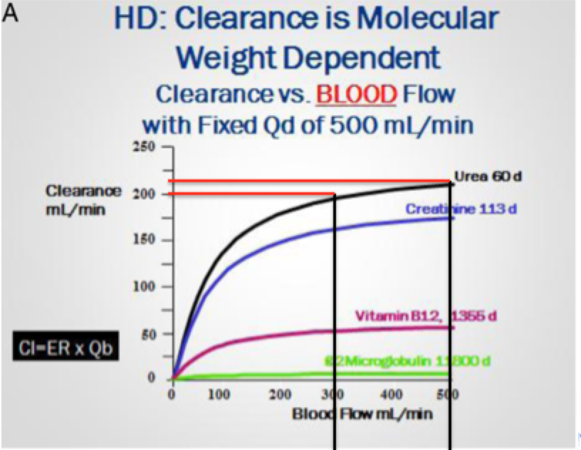
Both [1] and [2] canvas vitally important concepts in the practice of good, thoughtful, and protective access management. Both [1] and [2] speak loudly against thoughtless and ill-advised ramping of pump speed to achieve blood flows in excess of (my arbitrary maximum of) 350 ml/min. And—especially [2]—demonstrates the ever-diminishing returns to clearance from an ever-increasing pump speed; i.e. the law of diminishing returns. But, as the late-night TV ad-man so often says, “but wait – there’s more!”
Micro-bubbles
Micro-bubbles are just what they sound like: small, almost microscopic air bubbles that can—and do—form at several key sites within the extracorporeal circulation of a dialysis system.
A much-admired friend, André Stragier, a life-time dialysis technician from Belgium, now retired, but still a true sage of our profession, recently read Henning Söndergaard’s August 15, 2019 post, We Need a Revolution in Dialysis Treatment. Upon reading Henning’s impassioned plea, André was prompted to email me and remind me of (1) the importance of micro-bubble formation (MBF), and (2) the easy potential to revolutionize patient “risk” by ensuring a reduction in—or abolition of—MBF.
It appears that MBF relates directly to all of the blood flow issues I have previously discussed. Putatively, at least, there are three primary risk foci for MBF:
The higher the negative arterial pressure, the greater the risk of MBF.
The greater the blood flow rate, the greater the risk—and actuality—of bubble-trap MBF.
The hastier and more incomplete the priming—or perhaps with the “snap and tap” priming technique used with the NxStage system, the more agitation, the greater the risk of MBF.
I will quote, and at times paraphrase, part of André’s email to me:
“...more than 20 published papers reference the harmful effects of microbubbles in hemodialysis patients.
In summary: microbubbles enter undetected into the patients via the venous bloodline and lodge in various sites, but mainly in the lungs. These microbubbles obstruct blood flow when they lodge in the capillary circulation, and cause tissue ischemia and an inflammatory response through complement activation.
Aggregation of platelets and clot formation then occurs, leading to microcirculatory and tissue damage.
We should therefore—ASAP—seek to design systems that provide microbubble-free dialysis.”
This was André’s immediate sense of a “revolution;” one that would be so easy to implement, if only manufacturers would put their hearts and minds to work. While the full text of Andre’s attachment is provided as an addendum at the end of this post—with his permission—and all should take the few minutes required to read it, I will summarise the three main sites of microbubble formation he describes in his addendum:
Problem #1: The Arterial Lines Luer-lock Junction
A luer-lock connects the arterial needle to the arterial line of the dialysis line set. While this junction seems outwardly sealed and secure, the dialysis machine creates a negative pressure inside the blood tubing as it sucks blood from the fistula towards the dialyser.
The faster the pump speed, the greater the negative pressure in the tubing.
The greater the negative pressure, the greater the differential pressure between the outside room air and the blood. While we might like to think the luer-lock seal is a good one, small bubbles of air can—and do—"micro-leak” from the outside environment into the blood compartment. See the many references in André’s addendum. In a study by Wagner et al. [4.1] the microbubble rate upstream from the luer-lock connection was measured at 2 micro air bubbles/min, while downstream it was 46 micro air bubbles/min. The higher the negative arterial pressure, the more microbubbles were seen.
Solution: Devise an Air-seal for Both Luer Connections
Using simple silastic or gel technology, low-cost snap-caps (or similar) could be designed to externally seal and protect the negatively pressurized blood compartment from any externally-entering air. Even more basic might be to seal the luer-connection with impervious tape, like a small sliver of duct-tape (or similar). Simple solutions, yes—but yet to be considered.
In addition, smaller fistula needle sizes are also associated with higher negative pressures, in turn generating a higher rate of MBF. The same applies to catheter size.
Problem #2: The Venous Bubble-trap
Most conventional dialysis systems interpose a venous bubble trap after the dialyser to “catch” any air in the system and prevent it from returning with the venous blood to the venous limb of the fistula. The problem is the standard design of the venous bubble trap!
When the bloodstream enters the bubble trap, it carries along with it a small amount of the air present in the chamber top. This “carried” air is pulled downwards with the stream of blood entering from the top. As the upward “lifting force” of the microbubbles is less than the downward “driving force” of the stream of blood, small microbubbles travel lower into the bubble trap chamber.
This effect is magnified either by a low blood level in the venous chamber or by a high blood flow—or worse, both—and at high pump speeds, microbubbles are inevitably pushed through the bubble trap and into the venous return line.
Again, this is driven, I part, by that bête-noir word of any dialysis system: turbulence!
Solution: Devise a More Protective Bubble-trap
André’s team in Belgium solved this by redesigning the blood inlet in the venous chamber to position the return conduit 2 cm lower than usual, with the blood level 1 cm higher than the inlet. The cost of their redesigned lines was identical to that of the standard blood lines.
Problem #3: Retained Air from the Priming Process
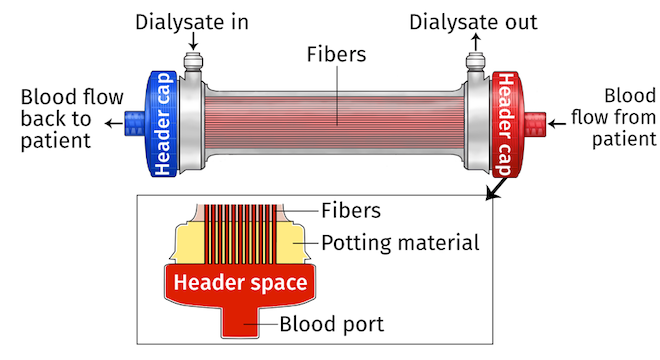
During dialyser priming, all of the hollow fibres of the dialyser should simultaneously and equally fill with priming saline. In practice, this doesn’t always work as well as it should. As priming saline takes the path of least resistance through the central fibres of the dialyser, some of the lateral fibres may retain air and contribute to microbubble formation, especially at the treatment start.
Solution: Add a Back-filter Phase to the Priming Process
Once the dialyser is thought to be adequately “primed,” add a 200-300 ml phase of back filtration (as per HDF) during which the positive pressure and flux will be identical for all fibres. This will remove any residual air. This step would demand ultra-pure dialysis fluid, but as ultra-pure dialysate should be part of all good dialysis, this should not present an insurmountable problem.
It should be of interest that an invited editorial in the International Journal of Artificial Organs, Matsuda K, Fissell R, Ash S, and Stegmayr B. “Long-Term Survival for Hemodialysis Patients Differ in Japan vs Europe and the USA. What Might the Reasons Be? doi: 10.1111/aor.13363. Epub 2018 Nov.11 has suggested just this: high blood flow rates are unavoidably associated with more microbubbles!
In summary, while it seems certain that MBF matters, we have far too little information on just how much microbubbles matter, or how much we should be worried about them. But, as they can be prevented—or minimised—by simple steps, should we not try to find out more of their consequences?
What does seem certain is that faster blood flow rates lead to greater micro-bubbling! So, as I cannot think that microbubbles in the return circulation are good for patients, I can (and will) add the risk of microbubbles to my crusade for gentler, softer, kinder dialysis…gentler, softer, and kinder to the fistula, and gentler, softer, and kinder to the patient. As Henning Söndergaard said in his August 15th post...we need a revolution in dialysis therapy, and this might just be one of the important battles to wage.
References
Matsuda K, Fissell R, Ash S, and Stegmayr B. “Long-Term Survival for Hemodialysis Patients Differ in Japan vs Europe and the USA. What Might the Reasons Be? ... see: doi: 10.1111/aor.13363. epub 2018 Nov.11
Microbubbles and Blood-Air Interface Prevention in Haemodialysis Patients
André Stragier, Dialysis Technologist (retired), UCL St. Luc-Renal Unit, 1200 Brussels, Belgium
Different authors (1,2) reported on the chronic presence of micro-air bubbles in the blood circuit of dialysis patients; they pass undetected through the bubble catcher air detector of the venous line (3,4). The clinical damaging consequences were clearly described (5,6,7 8): Micro air bubbles impede the blood circulation mainly in the lung capillaries: this causes tissue ischemia followed by inflammation and complement activation. There is an urgent need to solve this problem but so far only one dialysis company took measures that solved this problem partially (9).
There are three micro air bubble pollution sources to tackle:
Tighten the micro bubble air leakage of the arterial luer-lock connector:
Wagner et al. (1) measured in the arterial line 2 micro air bubbles per min. upstream of the luer-lock connector compared to 46 downstream from it; the higher the negative arterial pressure, the more microbubbles are reported.
In practice, when technicians connect two gas pipes they always use a seal, avoiding any leakage. However, in dialysis, the two connecting parts of the luer-lock connection are of hard plastic without seal. Their air tightness relies on the contact surface smoothness and the pressure with which the male part of the connector is pushed into the female one, and in practice, this screwing force varies between nurses and also for the same nurse from day to day. Incidentally, upon disconnection, the luer-lock can be stuck, needing two clamps to unscrew. When a central vein catheter us used, this can be damaging, necessitating catheter replacement.
One must realise that the viscosity difference between blood and air is huge: 4 versus 0.018 centipoise, respectively (10). This is why there is no blood leakage at the venous luer-lock connector (positive pressure) whereas at the arterial luer-lock side, a variable amount of micro-air bubbles are sucked into the bloodstream (negative pressure). This problem exists since more than 50 years but was only recently discovered (1).
This mechanical error needs to be urgently corrected! The solution is simple: add a mini-seal to the arterial luer-lock connector. This can for example be done by adding a mini film of slightly flexible Teflon or PTFE on the outside wall of the male connector, acting as a seal upon insertion into the female connector. This should also avoid the luer-lock being stuck upon disconnection. There is some small R&D work to find the best solution, suitable for automatic production. The effect of this improvement must of course be evaluated. The final goal is to obtain a completely air bubble free extracorporeal circuit. The problem of the arterial luer-lock leakage must absolutely be solved as this is most likely the most important problem of the three discussed in this text.
Avoid the micro air bubble generation and blood-air interaction in the bubble catcher.
When the blood stream falls in the bubble catcher, it takes with it a minor part of air present in the chamber top: a very small fraction of it is conducted with the stream because the lifting force of micro bubbles is lower than the driving force of the blood stream. This was repeatedly registered and the results revealed that this problem is the worst with a low level in the venous chamber or with a high blood flow (1, 12, 13). By opportunity, in 1995, we solved this problem and applied this solution in our unit in the UCL. First, we did so in our CVVH patients in the ICU to reduce the coagulation of the venous bubble catcher, thus reducing the frequency of the necessity to replace the whole circuit.
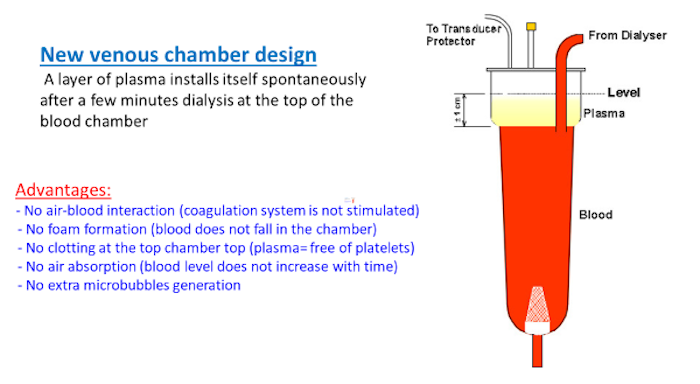 After this positive experience, this principle was extended to maintenance dialysis patients. We illustrate the way how this succeeded: the blood inlet in the venous chamber is positioned 2 cm lower than usually with the blood level 1 cm higher than the inlet. This offers several advantages, cited at the left hand side of the illustration. The cost of these lines was identical to that of the standard blood lines (gently offered by a small competing company!).
After this positive experience, this principle was extended to maintenance dialysis patients. We illustrate the way how this succeeded: the blood inlet in the venous chamber is positioned 2 cm lower than usually with the blood level 1 cm higher than the inlet. This offers several advantages, cited at the left hand side of the illustration. The cost of these lines was identical to that of the standard blood lines (gently offered by a small competing company!).
Eliminate all air bubbles during the dialyser priming
The problem of the dialyser capillaries is that they are in parallel position. In theory, all capillaries should be simultaneously and equally filled (principle of communicating vessels) during the slow priming with the venous outlet upside. But, in practice, this seems not to work perfectly, although it is not clear why. As soon as the capillaries are filled, the saline takes the pathway of the lowest resistance, i.e. through the central fibers, while in some other fibers air bubbles still remain; later on, in the beginning of the dialysis, they leave and become part of the air micro bubble problem. This can explain why more micro bubbles were measured at the beginning of dialysis compared to later and that wet stored dialysers entail less micro bubbles. (1,14). However, it is possible to resolve this problem during priming; as soon as the chambers are filled, we can include a phase of back filtration, some 200-300 ml: the positive pressure and the flux is identical for each capillary and, in this way, we can remove the residual air out of all capillaries. Therefore, one must dispose of ultra-pure dialysate, which in anyway is the preferred option, as it has the potential to decrease inflammation (14).
What are the advantages of microbubble and blood with air-contact free dialysis?
Medical: Discontinuing the chronic damaging of mainly the lung capillaries of our dialysis patients by micro air bubbles. Stopping its associated inflammation and complement activation may have a positive effect on the patients’ general health and survival, but, of course, this needs to be further evaluated.
Technical: Avoiding micro air bubbles pollution in the arterial blood line contributes to less clotting of the dialyser capillaries with better preservation of the dialyser clearance: this can be comparatively measured by the online clearance monitor. And, as an indirect consequence, avoiding the luer-lock to be stuck upon patient disconnection.
Hematologic: This also reduces the anticoagulation need (interesting for patients with a low anticoagulation prescription): Contact of blood with air stimulates the coagulation process. A high dose of anticoagulation is needed to prevent this. A venous bubble trap without blood-air interface, in our hands, was associated with less clotting in CVVH patients in the ICU with anticoagulant restriction and with a 33% heparin reduction (9).
References
Wagner S, Rode C, Wojke R, Canaud B. Observation of microbubbles during standard dialysis treatments. Clin Kidney J. 2015 Aug;8(4):400-4. doi: 10.1093/ckj/sfv051
Rollé F, Pengloan J, Abazza M, Halimi JM, Laskar M, Pourcelot L, Tranquart F. Identification of microemboli during haemodialysis using Doppler ultrasound. Nephrol Dial Transplant, 2000 Sep;15(9):1420-4.
Jonsson P, Karlsson L, Forsberg U, Gref M, Stegmayr C, Stegmayr B. Air bubbles pass the security system of the dialysis device without alarming. Artif Organs 2007 Feb;31(2):132-9.
Keshavarzi G, Barber TJ, Yeoh G, Simmons A, Reizes JA. Two-dimensional computational analysis of microbubbles in hemodialysis. Artif Organs 2013 Aug;37(8):E139-44. Doi: 10.1111/aor.12110
Forsberg U, Jonsson P, Stegmayr C, Stegmayr B. Microemboli, developed during haemodialysis, pass the lung barrier and may cause ischaemic lesions in organs such as the brain. Nephrol Dial Transplant 2010; Aug;25(8):2691-5. Doi: 10.1093/ndt/gfq116
Barak M, Katz Y. Microbubbles: pathophysiology and clinical implications. Chest 2005 Oct;128(4):2918-32
Brännström T, Forsberg U, Jonsson P, et al. Microembolies of air are deposited in the lungs of heamodialysis patients. Int J Artif Organs 2012; 35: 579
Stegmayr B, Brännström T, Forsberg U, et al. Microbubbles of air may occur in the organs of hemodialysis patients. ASAIO J 2012 Mar-Apr;58(2):177-9. Doi: 10.1097/MAT.0b013e318245d0dd
Introductory primer Streamline: Airless, simple, the new standard in blood tubing
ULF Forsberg. Presence of microemboli during haemodialysis and methods to reduce the exposure to microbubbles
Forsberg U, Jonsson P, Stegmayr C, Stegmayr B. A high blood level in the air trap reduces microemboli during hemodialysis. Artif Organs 2012 Jun;36(6):525-9. Doi: 10.1111/j.1525-1594.2011.01415.x
Forsberg U, Jonsson P, Stegmayr C, et al. A high blood level in the venous chamber and a wet-stored dialyzer help to reduce exposure for microemboli during hemodialysis. Hemodial Int 2013 Oct; 17(4): 612–7.doi: 10.1111/hdi.12052
Ledebo I. Ultrapure dialysis fluid--direct and indirect benefits in dialysis therapy. Blood Purif. 2004;22 Suppl 2:20-5.


Comments
Morgan
Sep 03, 2023 7:33 AM
Debra Null
Sep 03, 2019 6:59 PM
I also have always been uneasy with the way we are taught to set up and prime the NxStage machine. One of the things that I don't get is that the arterial and venous lines are instructed to be connected by a female-female connector that is about 1 1/2 inches long and that air then mixes with the solution in the lines. I've always been told..."oh don't worry about that, you can never get all the air out." Somehow I was not reassured.
John Agar
Sep 04, 2019 12:16 AM
André Stragier
Sep 03, 2019 3:11 PM
1. Air microbubbles are currently sucked into the bloodstream and activate the complement and the clotting system.
2. Next, a small part of these microbubbles are trapped in the dialyser capillaries and enhance clotting.
3. The remaining microbubbles pass through the dialyser, through the venous bubble trap into the patients lungs; there they impede the blood circulation in some of the lung capillaries causing tissue ischemia followed by complement activation and inflammation.
This happens unnoticed since more than 50 years, and was only recently discovered. This is now a clear, new item for an important dialysis quality improvement. Optimal dialysis should be microbubbles and air-blood interface free (see my technical note above). Therefore, we ask all our industry dialysis partners to helping us ASAP!
John Agar
Sep 04, 2019 12:07 AM
I can only hope that some in industry may read this blog and come to the party with the design and manufacture of smart ways to secure an airtight arterial luer-lock connection.
It shouldn't be overwhelmingly difficult or expensive to do.
Fingers crossed!
Ant
Aug 30, 2019 10:38 PM
With erythrocytes approx. equal in diameter to micro-bubbles its unnerving to visualize them sharing the plasma stream with hordes of them.
Three things I'll implement next dialysis session; venous bubble trap level raised to maximum, strip of masking tape tightly wrapped round arterial luer, even slower Qb.
John Agar
Aug 31, 2019 7:22 AM
Re the 'masking tape' idea ... that option is un-tested and you will be an 'n' of one ... it just seemed to me to be an idea that couldn't harm, and might just reduce the risk. But, a better solution shouldn't be complex.
As for the other two, I think the bubble-trap issue is important, and there is little point raising the flow rate over a max of 325-350 ml/min. The graph shows that a large rise in blood flow [on horizontal axis] from (say) 300-500 yields a minimal clearance benefit [on the vertical axis] for urea ... (the black curve), even less for creatinine (the red curve) and damn all for larger molecular weight solutes. All very fast flow rates do is to raise the risk of bruising and battering red cells in the roller pump, raise the risk of sucking in micro-bubbles, and creating excessive venous limb turbulence as blood jets back into the vein.
Ant
Sep 01, 2019 12:20 AM
I was unable to find whether the Yu-ki Ban tape is gas permeable. I’d intended to use it to seal the arterial luer. As for the difficulty recorded undoing the lock once ultra tightened (and with tape twisted round it) - this could readily be overcome by snipping the line downstream from the clamp with scissors.
John Agar
Sep 02, 2019 12:41 AM
André Stragier
Aug 31, 2019 7:28 PM
Sofie Jamar
Dec 01, 2020 3:23 PM
Are you aware of these differences between machines and are dialysers with casette system safer in general?
Henning Sondergaard
Aug 29, 2019 9:25 PM
On a more personal level I am blown away by the fact that my (completely unscientific) opinion piece leads two great minds to this. I love it when such serendipitous events happen.
Theodôr
Aug 30, 2019 7:06 AM
I feel the extensive and clear outlined risks of high pump settings are excellently outlined by John! Chapeau for that.
I would name this getting the knowledge and "feel" accross on "MINDFULL Dialysis"! Cheers Theodôr