ESRD Payment Rule Under Original Medicare for CY 2025

ESRD PPS Base Rate & Adjustments
CMS published the ESRD Prospective Payment System (PPS) Final Rule on November 1, 2024. The provisions in this document take effect January 1, 2025, so here’s a summary of the main ones. CMS will pay $273.82 for bundled payment claims to Original Medicare. NOTE: Dialysis clinics must bundle dialysis and ESRD-related labs and drugs for patients with Original Medicare. However, clinics do not have to bundle services on claims to Medicare Advantage, Veterans, Marketplace plans, and commercial payers. These entities do not require claims to be bundled, and dialysis clinics can bill those items separately.

The ESRD PPS continues to have patient level adjustments, including age, low body surface area and low body mass index, and comorbidities (chronic and acute). The onset of dialysis adjustment still pays more for the first 120 days of treatment. There are also facility level adjustments to the ESRD PPS for geographic wage differences, clinics in rural areas, clinics that provide fewer than 4000 treatments a year, and for training. Medicare doesn’t pay extra for comorbidities or home dialysis training when it’s paying the onset of dialysis adjustment, but will adjust for them after it stops paying the onset of dialysis adjustment.

Home Dialysis Options for Patients with Acute Kidney Injury
There is exciting news for patients with AKI who want to do home dialysis! CMS is revising the Conditions for Coverage (regulations), and Medicare will cover home dialysis training and home dialysis treatments (PD or home HD) for patients diagnosed with AKI. Previously Medicare covered only in-center HD for those with AKI.
Phosphate Binders in the ESRD PPS
The BIG news is that effective January 1, 2025, CMS will include phosphate binders in the ESRD PPS (“bundle”); a change that has been in the works for more than a decade. Initially CMS added oral drugs to the ESRD bundle when there was an IV equivalent, and proposed to add oral-only ESRD-related drugs (phosphate binders) in 2014. However, in 2012 a law was passed that delayed the addition of oral-only drugs until 2025.
CMS plans to use the process it followed in 2018 to add oral cinacalcet (Sensipar) and IV etelcalcetide (Parsibiv) to the ESRD bundle. It will pay clinics a Transitional Drug Add-on Payment Adjustment (TDAPA) for at least 2 years. The 2025 amount of $36.41 will be added to claims that include a phosphate binder. For more information on this process, see the CMS publication Including Oral-Only Drugs in the ESRD PPS Bundled Payment. The recently approved twice a day phosphate blocker XPHOZAHTM will be in the bundle. If Ardelyx, the manufacturer, wants TDAPA payment, it will need to apply for a code and TDAPA. Here are HCPCS codes for claims with phosphate binders for TDAPA payment:

J0601, “Sevelamer carbonate (Renvela or therapeutically equivalent), oral, 20 mg (for ESRD on dialysis)”
J0602, “Sevelamer carbonate (Renvela or therapeutically equivalent), oral, powder, 20 mg (for ESRD on dialysis)”
J0603, “Sevelamer hydrochloride (Renagel or therapeutically equivalent), oral, 20 mg (for ESRD on dialysis)”
J0605, “Sucroferric oxyhydroxide, oral, 5 mg (for ESRD on dialysis)” o J0607, “Lanthanum carbonate, oral, 5 mg (for ESRD on dialysis)”
J0608, “Lanthanum carbonate, oral, powder, 5 mg, not therapeutically equivalent to J0607 (for ESRD on dialysis)”
J0609, “Ferric citrate, oral, 3 mg ferric iron, (for ESRD on dialysis)”
J0615, “Calcium acetate, oral, 23 mg (for ESRD on dialysis)”
For any other oral renal dialysis drug or biological product that is not assigned a HCPCS code, ESRD facilities would report the NDC of the drug on the claim form.
The amount paid under TDAPA is based on 100% of the drug manufacturer’s average sales price (ASP). If the ASP is not available, CMS bases TDAPA on 100% of the wholesale acquisition cost (WAC) or the drug manufacturer’s invoice. When the TDAPA period ends, CMS will adjust the ESRD PPS amount for the following 3 years for all dialysis claims, including those for patients not on binders. The amount of the increase will be based on cost and drug utilization for the previous 12 months. For more information, see CMS Transmittal 12962.
Industry Concerns about Adding Binders to the ESRD PPS
Dialysis providers and kidney organizations advocated with Congress and CMS not to include phosphate binders in the bundle. A 2023 General Accounting Office report stated that according to the United States Renal Data System (USRDS), 60% of ESRD patients with Part D were prescribed a phosphate binder: 62% took sevelamer and 35% took calcium acetate—despite KDIGO guidelines recommending limited use of calcium-based binders to avoid blood vessel calcification. The GAO interviewed 5 large dialysis organizations that treat over 85% of dialysis patients and an association representing 120 small and independent dialysis providers, and their concerns included:
Numbers of prescriptions for binders will be much greater than for other bundled drugs.
Dispensing is complex, with multiple binder options, doses, and changing patient needs.
Technology updates will be needed along with hiring more nurses, pharmacists, and administrative staff.
More space for secure storage will be required to meet state regulations if binders are shipped to the clinic vs. patients’ homes.
Guidance for prescribers is needed that allows them to prescribe the binder the patient needs while being attentive to costs and savings.
After the TDAPA period ends, if CMS revises the bundled payment using the same process as for calcimimetics, the increase may not account for all costs, especially for smaller providers who lack the buying power to get lower cost binders like large dialysis organizations.
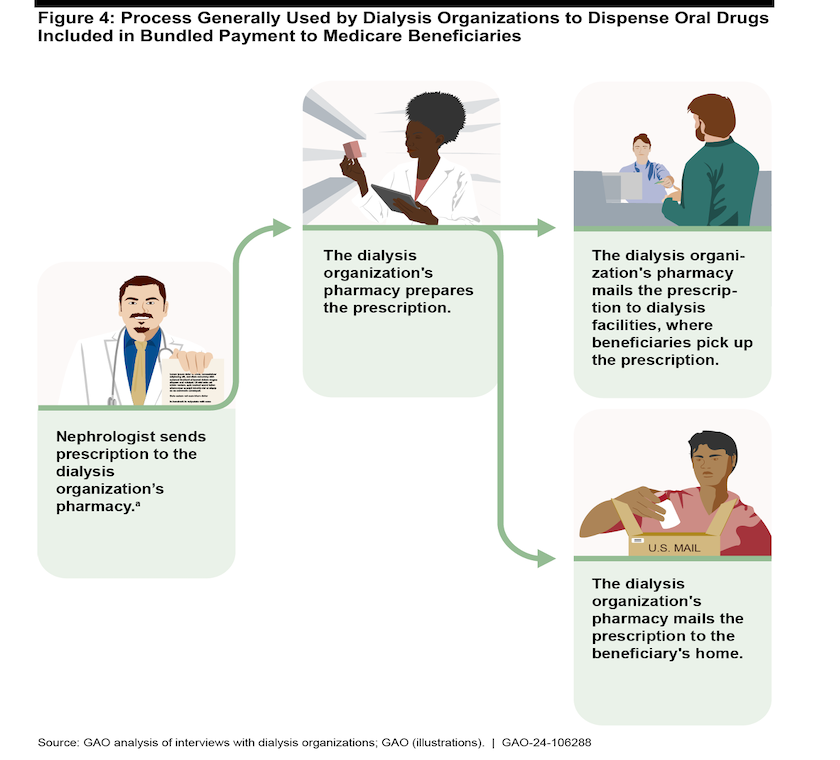
A GAO graphic illustrates the process large dialysis organizations use to get oral drugs to patients. Smaller providers may not have their own pharmacies and will need to contract with a pharmacy to dispense drugs to patients.
Despite Complexity, Why Binders in the PPS May Be Good for Patients
Per the USRDS 2022 Annual Data Report, an estimated 60% of all Medicare patients used Part D. However, 20% of ESRD patients didn’t have Part D, which may have limited their ability to take prescribed drugs—including phosphate binders. When ESRD-related drugs are in the ESRD bundle, Medicare Part B covers them along with the dialysis treatment and ESRD-related labs. Once a patient has met the Medicare Part B annual deductible ($257 in 2025), as a primary payer Medicare will pay 80% of allowed charges for covered services. The remaining 20% can be paid in full or in part by a Medicare supplement (Medigap), Medicaid, or job-based plan.
A Dialysis Provider’s Thoughts on This Change
 Jeffrey Giullian, MD, MBA,
FASN, the chief medical officer for DaVita Inc. recently wrote an
article
for the November 2024 issue of Healio Nephrology News &
Issues. In it, he discussed the importance of preparing
patients and nephrologists through education about the upcoming
change. Giullian disputes speculation that providers would
limit binders to only lower cost calcium-based ones and said DaVita has
been working on this change for some time. In fact, he said
DaVita’s plan is to have at least one agent from each class of binders
so nephrologists can prescribe what’s best for each patient.
Without having to worry about Part D formularies and copays, he expects
DaVita patients to have better access to iron-based binders. He
said, “This change has the potential to improve patient care and
potentially reduce pill burden.”
Jeffrey Giullian, MD, MBA,
FASN, the chief medical officer for DaVita Inc. recently wrote an
article
for the November 2024 issue of Healio Nephrology News &
Issues. In it, he discussed the importance of preparing
patients and nephrologists through education about the upcoming
change. Giullian disputes speculation that providers would
limit binders to only lower cost calcium-based ones and said DaVita has
been working on this change for some time. In fact, he said
DaVita’s plan is to have at least one agent from each class of binders
so nephrologists can prescribe what’s best for each patient.
Without having to worry about Part D formularies and copays, he expects
DaVita patients to have better access to iron-based binders. He
said, “This change has the potential to improve patient care and
potentially reduce pill burden.”
Final Questions & Conclusion
Are other dialysis providers and nephrologists as prepared and forward thinking as Dr. Giullian? Can CMS collect the data needed to calculate an amount to increase the ESRD bundle to cover clinics’ costs to acquire, store, and dispense phosphate binders—especially for smaller and independent dialysis providers? If so, this change may increase patients’ access to phosphate binders and avoid the discomfort and complications of renal bone disease. Only time will tell.


Comments
Amanda Gabel
Aug 27, 2025 4:43 PM
JB
Sep 30, 2025 4:26 PM
Have you received any update on this? I am having this issue as well. BCWV stated that we should not be billing with AX modifiers with the phosphates, but I am not 100% certain if this is the case under all Highmark plans and am unable to locate the information.
Thanks!
Beth Witten
Sep 30, 2025 8:00 PM
If you read the text in the email message I received from CMS when I asked whether the ESRD PPS applies to Medicare Advantage plan, I was told it doesn't. So, my interpretation is that dialysis patients who have MA plans will need a prescription and should get their phosphate binders from their MA plan's network pharmacy using their MA card like they get their other prescribed drugs.
Beth Witten
Sep 08, 2025 6:10 PM